Pharmaclean® innovation for sterilization processes: a new envelope 100% aligned with Annex 1 requirements
Conversation with Annalisa Soldi, Product Manager Contamination Control AM Instruments

A few weeks after CPhI 2024, where AM Instruments unveiled the world premiere of the new Pharmaclean® envelope, can we better understand what innovations this product brings?
"The AM Pharmaclean® line is designed to stand out in the market with quality offerings that aim to improve operational efficiency and innovate production processes. The new Tyvek®-PET/PP pouch not only fully meets Annex 1 requirements, but elevates them to the next level. In addition to ensuring contamination control and compliance, this pouch introduces a unique innovation in pharmaceutical packaging.
The process indicator, printed with validated technology, ensures the absence of chemical residues during autoclave sterilization and immediate verification of the outcome of the process.
In addition, printing the lot number and product code enables full traceability and reduces the risk of cross-contamination, optimizing post-sterilization product management."
One year after Annex 1 went into effect, how does Pharmaclean® respond to the need to reduce the risk of contamination in the pharma industry?
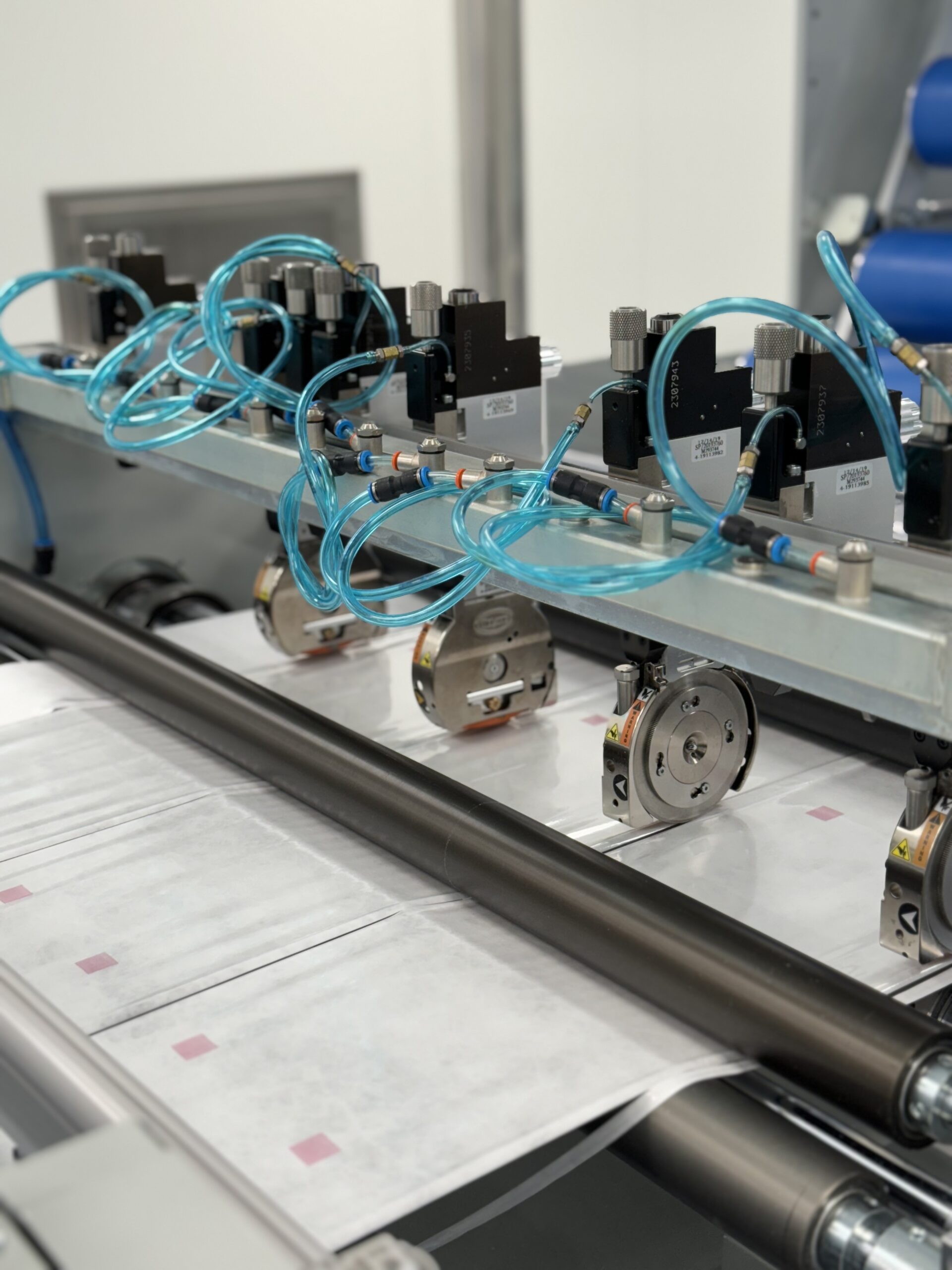
"Our Pharmaclean® production has been made in compliance with the challenges introduced by the new Annex 1, starting with material selection. Tyvek® is the ideal choice for solutions intended for packaging, transportation and sterilization of equipments, due to its resistance to tearing and puncturing, low particle release and ability to allow uniform steam distribution during autoclaving.
In addition, the entire production process takes place in Grade C operational cleanroom environments. Every step, from raw material entry to finished product release, is tracked and documented in dedicated batch records, and the classified environment is monitored at the particle and microbiological level in accordance with UNI EN ISO 14644-1 and Annex 1 requirements.
But we do not stop at mere compliance. Our goal is to offer customers a cleaner finished product than individual raw materials. That's why both Tyvek® and PET/PP undergo treatment with the proprietary Safe4Clean® system, which enables the abatement of at least 60 percent of contaminants on the materials before they are processed. This approach allows us to go beyond regulatory requirements, ensuring unparalleled safety and quality for our customers in the pharmaceutical industry."
During the July integrated budget presentation, AM Instruments made the expansion of the production unit official. What are the future prospects?
"During the integrated budget presentation, we announced the expansion of our production unit in Limbiate. Currently, AM Instruments operates with two cleanroom, both Grade C operational; the first at our head quarter and the second in Cesano Maderno.
The expansion of the Limbiate cleanroom not only represents an increase in production capacity to meet growing market demand, but also allows us to ensure a fully integrated production process, from raw material processing to finished product, without intermediaries. This allows us to generate batches that are perfectly aligned with regulatory requirements.
In addition, we plan to open a new Quality Control (QC) laboratory, which will offer additional services to our asepsis customers. With this new facility, we will be able to meet customer needs with the utmost precision and ensure high standards of quality and traceability at every stage of the production process."
Pharmaclean® is known for its ability to customize solutions and the ongoing support offered to customers. How important is customization for success in the market?
"Customization is a key pillar of our strategy. Pharmaclean® is not limited to being a supplier; we position ourselves as a partner for our customers, offering ongoing support at every stage of the process, from design to final implementation. This consultative approach allows us to work closely with the customer, right from the feasibility studies, to identify together the solution best suited to their specific needs.
Our process begins with a thorough analysis, followed by the production of samples tested directly by the customer. Only after their approval do we proceed to final production and batch release, complete with all regulatory-compliant documentation. This approach not only strengthens the relationship with the client, but also ensures a customized solution tailored to the requirements.
Experience has taught us that suitable packaging in shape and size is a key deterrent against the risk of contamination in sterilization processes. Customizing packaging means contributing significantly to product safety and customer operational success, making Pharmaclean® a reliable partner for those in the industry."
Quality is a core value for AM Instruments: what does it mean to be GMP Consistent®?
"Since 2020, AM Instruments has been GMP Consistent® certified, and this standard represents more than just regulatory compliance. For us, being GMP Consistent® means taking an integrated approach to quality, involving every area of the company: from research and development to production, to customer service, logistics and technical support.
Innovation for us is not just about product, but a daily commitment to listening and understanding customer needs. For us, being GMP Consistent® is a sign of reliability and dedication to quality, a mark that represents our willingness to go beyond expectations and contribute to the success of our partners."